Polyelectrolytes
General Properties
Polyelectrolytes are polymers with dissociating groups in their repeat units. They can be divided into polycations and polyanions and polysalts. Like ordinary electrolytes (acids, bases and salts), they dissociate in aqueous solutions (water) and bear one or more charges depending on the pH value. Thus, the properties of polyelectrolytes are similar to both electrolytes and polymers. The salts, i.e. the products of a polyacids (polyanions) with a monomeric base and vice versa are called polysalts. Like regular salts, their solutions are electrically conductive and like polymers, their viscosity strongly depends on the molecular weight and polymer concentration.
The three most common anionic groups are carboxylate (–COO-), phosphonate (–PO3H-, –PO32-), and sulfonate (–SO3-) and the most common cationic groups are primary, secondary and quaternary ammonium (–NH3+, =NH2+ & ≡N+). The type of ionic group, its counter ion and the structure of the repeat unit determine the properties of a polyelectrolyte such as solubility in water and other polar and hydrogen-bonding liquids (alcohols etc.), electrical conductivity, and solution viscosity. Unlike nonionic polymers, these properties strongly depend on the pH and salt content.
Polyelectrolytes can be chemically crosslinked by incorporating a small amount of a suitable crosslinking agent. These polyelectrolytes form three-dimensional structures that swell in water rather than dissolving in it. They can retain (extremely) large amounts of liquid relative to their own mass through hydrogen bonding with water molecules. They are called hydrogels or superabsorbent polymers (SAP’s) when (slightly) cross-linked. Their ability to absorb water is a factor of the ionic concentration of the aqueous solution. In deionized and distilled water, SAPs may absorb water up to 500 times their own weight and from 30 to 60 times their own volume, that is, a hydrogel can consist of more than 99% liquid. The total absorbency and swelling capacity of SAP’s is controlled by the type and amount of crosslinks in the structure.
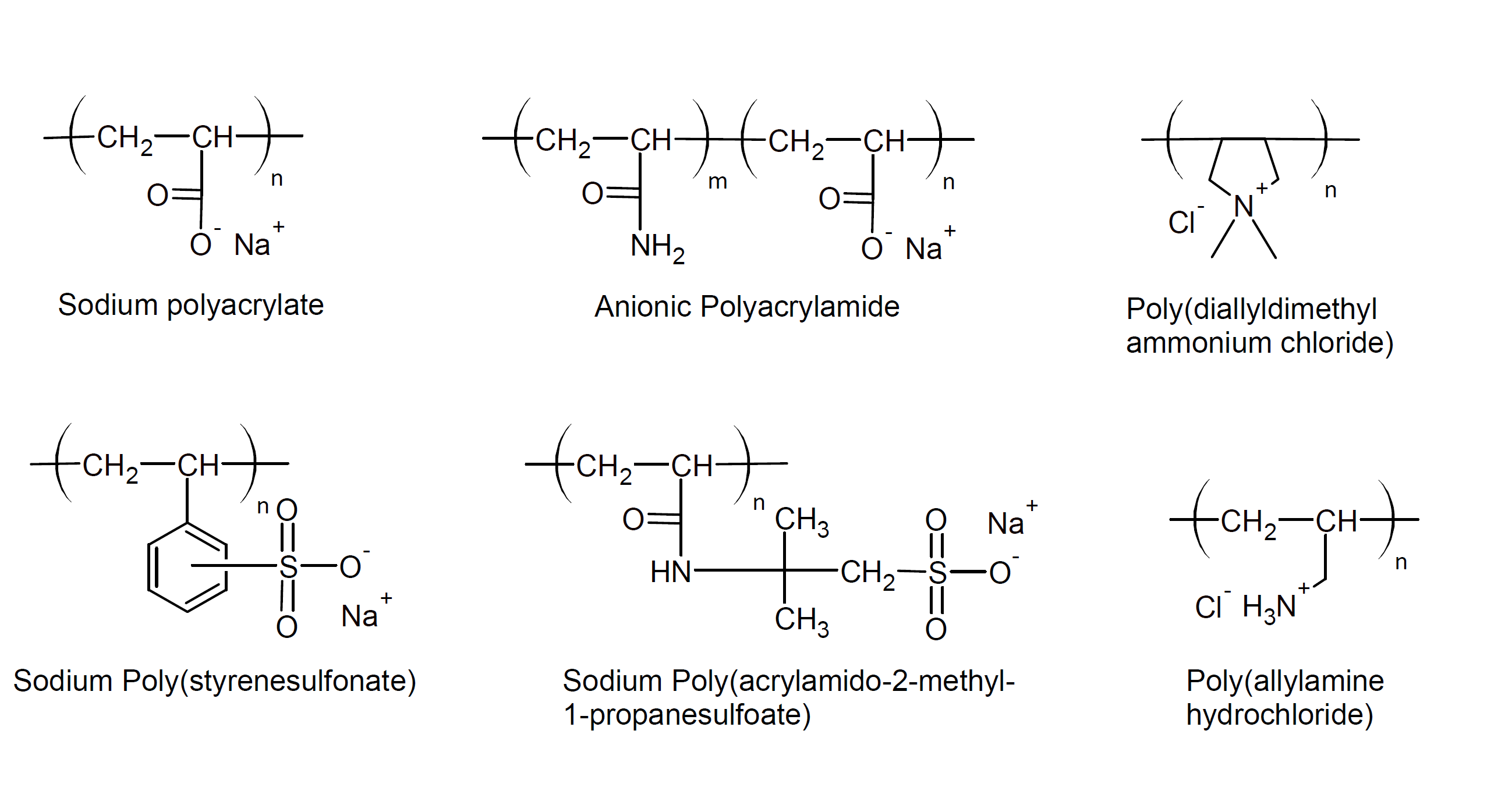
Both natural and synthetic polyelectrolytes are manufactured on a
large scale. Common natural polyelectrolytes are pectin
(polygalacturonic acid), alginate (alginic acid), carboxymethyl
cellulose and polypeptides. Examples of common synthetic
polyelectrolytes are polyacrylic acid, polystyrene sulfonate,
polyallylamine, carboxymethyl cellulose and their salts. Some of these polyelectrolytes are depicted below:
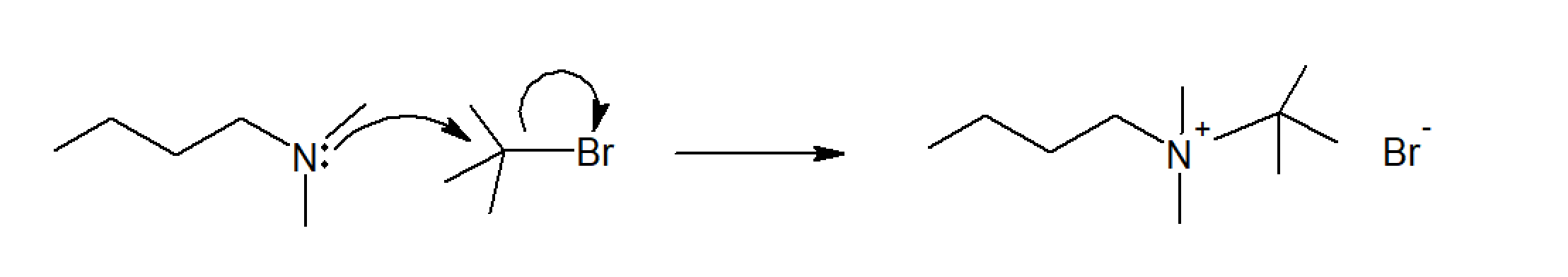
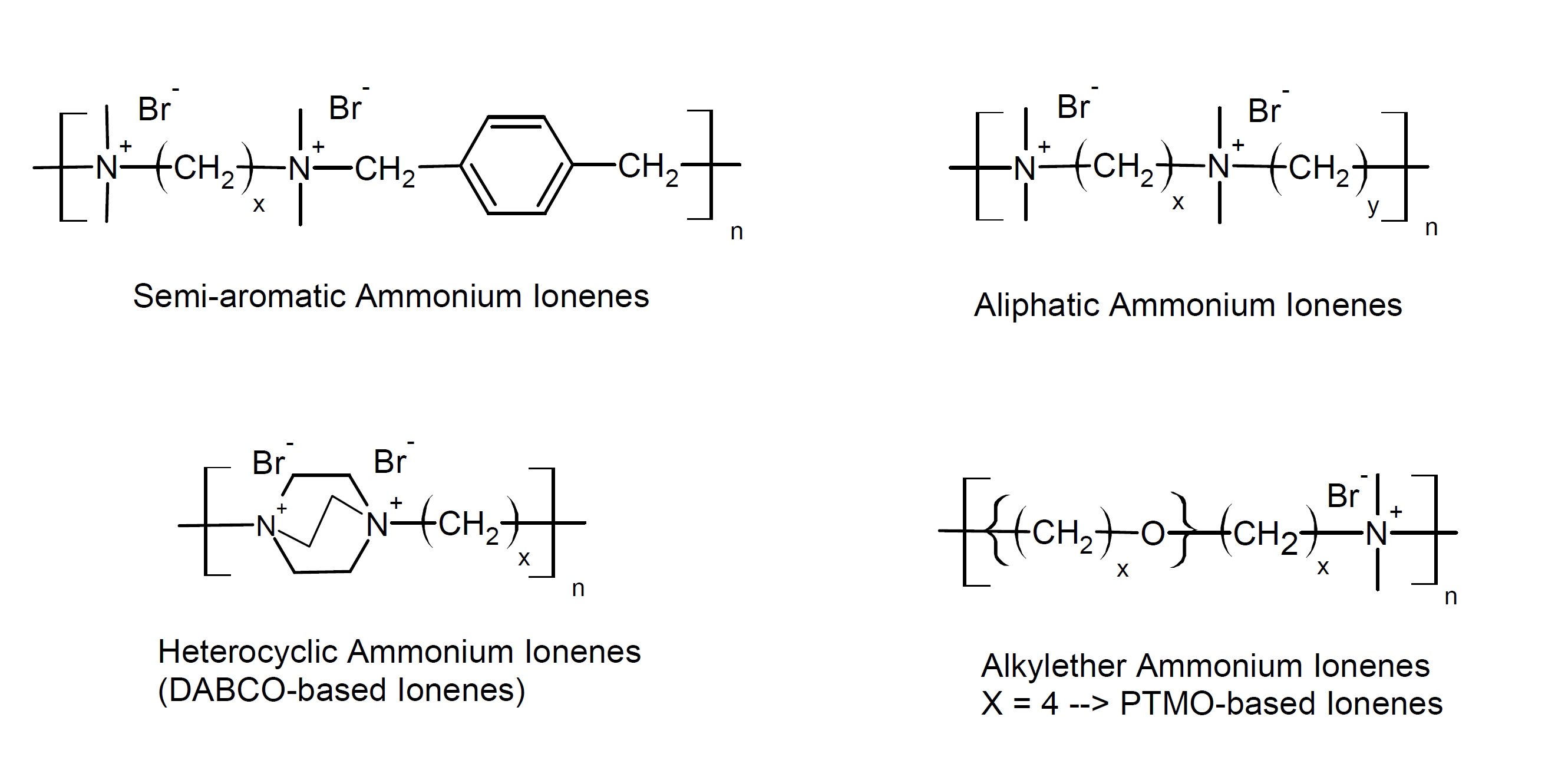
Some other polyelectrolytes contain charged groups in the main chain as opposed to charged side groups. This class of polyelectrolytes is commonly known as polyionenes. The most common charged group in the backbone is quaternary ammonium. These ionic polyamines are typically synthesized through a Menshutkin reaction of ditertiary amines and alkyl dihalides.
A large number of dihalides and ditertiary amines are commercially available which permits the synthesis of a large number of well-defined polyionenes from relative cheap starting materials and a relative simple synthesis route. Both the ionic spacing on the polymer backbone, i.e. the charge density, and the molecular weight can be easily adjusted. In addition, many other functional groups can be easily incorporated, which allows for crosslinking and tailoring the physical and mechanical properties.
Physicochemical Properties
The properties of polyelectrolytes in solution are determined by the electrostatic interaction between the charged groups in the chain and
the low-molecular-weight ions in the solution.
The strong electrostatic field generated by the charges in the polyelectrolyte has a significant effect on the structure of the molecule, that is, it substantially alters the macromolecular conformations;
with increasing degree of dissociation, the effective size of the chains (end-to-end distance, hydrodynamic radius, etc.) increases, and the coiled molecules straightens out reaching an approximately linear,
stick-like shape at high degrees of polyelectrolyte dissociation.
The physicochemical properties undergo considerable alteration
when the degree of dissociation changes. For example, the solution viscosity can increase by a factor
of a hundred or more depending on the polyelectrolyte concentration
in solution, its degree of dissociation and the free ion (salt) concentration (low-molecular-weight ions in the solution).
The theory that was developed for solutions of low-molecular-weight electrolytes is not valid for polyelectrolyte solutions. The low-molecular-weight ions that appear during the dissociation of polar groups of the polyelectrolytes create a diffuse counter-ion cloud around the oppositely charged surface of the polymers. Its composition (solvation) and size influences the structure and dynamics of polyelectrolytes in solution as well as the swelling capacity and visco-elastic response of polyelectrolyte gels.
Charged molecular chains commonly present in soft matter systems have a noticeable effect on the structure, stability and the interactions of various (biological) molecular assemblies. Theoretical approaches to describe their statistical properties differ profoundly from those of their electrically neutral counterparts. Many biological molecules are polyelectrolytes. For instance, polypeptides and DNA’s are polyelectrolytes.
COMMERCIAL Polyelectrolytes
Commercial grades of polyelectrolytes (PAA's) are available from Dow Chemical (Duramax, Tamol™, Romax™, Dowex), Rohm and Haas (Acusol™,Acumer™, BASF (Dispex®, Magnafloc®), and Arkema (Rheoslove™, Terrablend).
| Polyelectrolyte | Structure of Repeat Unit | Trade Name |
| Polyacrylic acid (PAA) |
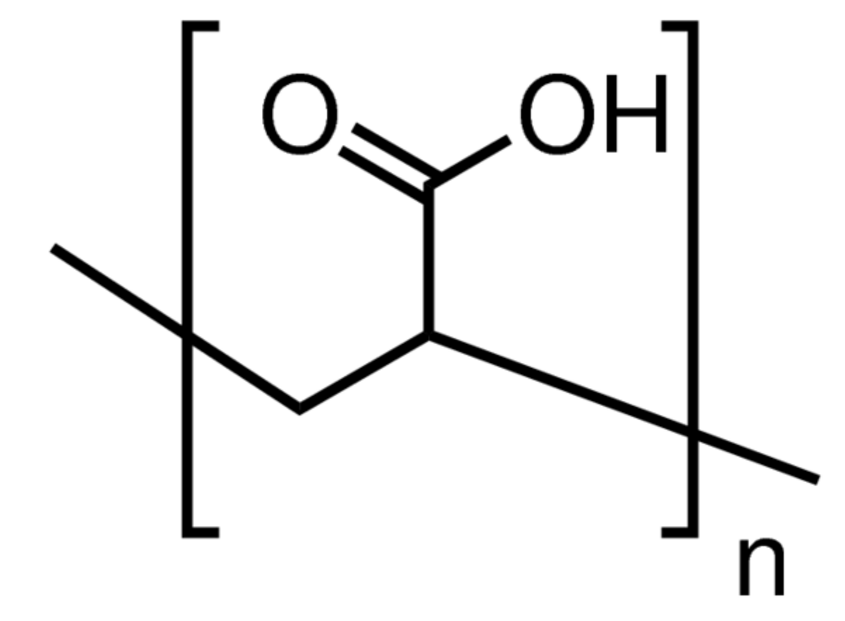
| Acusol (copolymer), Acumer, Acrysol, Alcosperse |
| Polystyrene sulfonate |
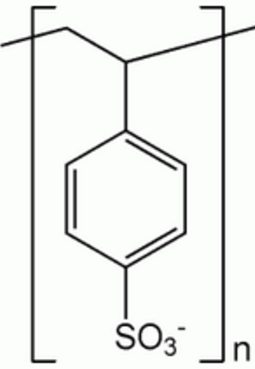
| Resonium, Sorbisterit, Resikali |
Applications
Polyelectrolytes have many applications, mostly related to modifying flow and improving the stability of aqueous colloids and gels or to induce agglomeration. For instance, they can be used to destabilize a colloidal suspension and to initiate flocculation and precipitation. They can also be used to impart a surface charge to neutral particles, enabling them to be dispersed in an aqueous solution. They are thus often used as thickeners, dispersant agents, conditioners, emulsifiers, ion-exchanger and clarifying agents. For example, they are used in water treatment as flocculation agents, in ceramic slurries as stablizing agents and in concrete mixtures as superplasticizers. Furthermore, many shampoos, soaps and cosmetics contain polyelectrolytes. Certain polyelectrolytes are also added to food products; for example, as food coatings and release agents. Examples are pectin (polygalacturonic acid), alginate (alginic acid), and carboxymethyl cellulose. All but the last are of natural origin.
Because polyelectrolytes are water-soluble, they are also used in biochemical and medical applications such as implant coatings and controlled drug release systems.
Large quantities of slightly cross-linked sodium polyacrylate-polyacrylamide copolymers, called hydrogels, are used as super absorbents (SAPs). The largest use of SAPs is found in baby diapers and other personal disposable hygiene products, such as adult protective underwear and sanitary napkins. The global super absorbent polymer market is expected to grow at a rate of 5.5% from 2014 to 2019 to reach a value of $ 8.56 billion.1
THERMO-PHYSICAL PROPERTIES
1Global Super Absorbent Polymers Market - Forecast to 2019, DUBLIN, July 2, 2014 - PRNewswire - Research and Markets
2The generic name ionene, which is the abbreviation
for ionic amine, was proposed by Rembaum et al. and has been widely accepted. (A. Rembaum et al., J. Poly. Sci. Part B: Poly. Let., 6(3), 159-171, 1968)